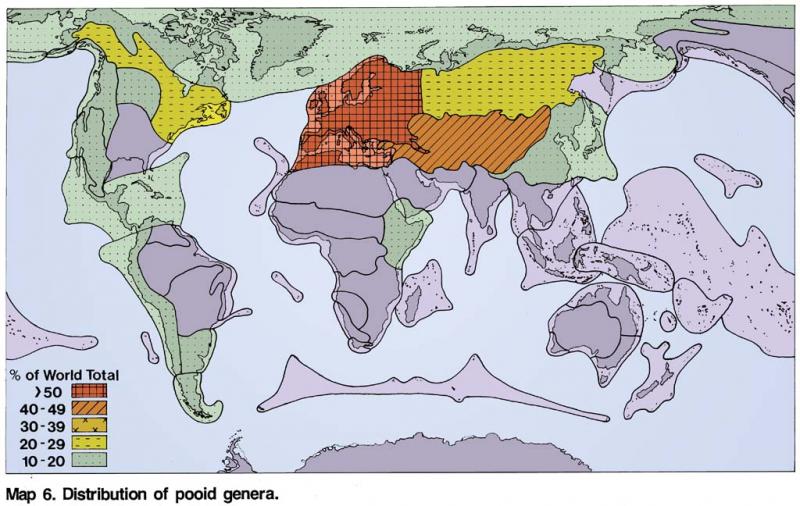
Pooideae. –This subfamily is the largest in the grass family consisting of 4200 species and 194 genera (Table 2) and occurs in all regions of the world with a temperate climate in high latitudes and altitudes. It includes some of the world’s most
prolific genera Festuca (635 species), Poa (544 species), Agrostis (219 species), Stipa (193 species), Bromus (160 species), Puccinellia (115 species), as well as many smaller and monotypic genera. Until the early 1980’s the Pooideae has probably been the subject of a greater number of classifications than any other
subfamily (Macfarlane, 1986; Macfarlane & Watson1980, 1982) and twelve such
classifications are mentioned and compared by Macfarlane & Watson (1982).
Up to this point all classifications were phenetically derived using morphology
and latterly anatomy; the most detailed of these is that of Macfarlane (1986),
where 157 genera, excluding the tribe Stipeae, are placed in seven tribes
within two supertribes Pooideae and Triticoideae. The classification of Clayton
& Renvoize (1986) included 153 genera in 10 tribes with their relationships
presented in three phylogenetic diagrams. An attempt to undertake a cladistic
study based on morphology in the Pooideae (Kellogg & Watson 1993), based on
189 genera, resulted in a lack of a hierarchy equivalent to traditional tribes.
Reasons given for this lack of resolution were a rapid burst of evolution in
the tribe, intergeneric gene flow and parallellism of morphological characters.
Early molecular studies, based on 33 species of 28 genera (Soreng et al., 1990)
did, however, produce a topology onto which 5 traditional tribes of the
subfamily could be superimposed. A cladistic study based on both 601 molecular
characters (chloroplast DNA) and 67 morphological characters (Soreng &
Davis, 2000), using 79 genera and 101 species of the Pooideae resulted in a
topology that blurred the distinction between the tribes Aveneae and the Poeae
to the extent that it was recommended that the barrier between these tribes be
abandoned, a proposal first suggested on non-phylogenetic principles (Tzvelev,
1989). Within this study the Bromeae, Triticeae, Meliceae, Brachypodeae and
Stipeae were shown to be monophyletic, although the sample size was far less
than that for the Aveneae/Poeae complex. Some of these results were
substantiated by Davis and Soreng (2007) and Soreng at al. (2007), and in the latter
paper the Poaeae sens. lat. (Aveneae/Poeae complex) are classified into 21
subtribes on the basis of their position in a cladogram derived from
morphological data and data from nucleotide sequence variation. Based on only
57 species and with no attempt to allocate diagnostic characters to the
subtribes, I am of the opinion the proposal of a new classification on the
basis of the data presented is premature. A similar study of the phylogenetic
relationship within the Aveneae/Poeae complex based on matK chloroplast sequence data (Doring et al 2007) verified the
undefined boundary between the genera traditionally allocated to these these
tribes and that a new generic circumscription of this group is probably
required before applying a subtribal classification.
Pooideae tribe Stipeae.
-- An examination of recent literature concerned with the composition and characteristics of the stipoid grasses reveals that the classification of this group is far from being resolved (Barber et al. 2009; Barkworth 1990; Barkworth 1993; Barkworth & Everett 1987; Jacobs & Everett 1996; Jacobs et al. 2000; Romaschenko et al. 2008a, Romaschenko et al. 2010). In the most recent study (Romaschenko et al. 2011) the genus Piptatherum was shown to be polyphyletic and two genera, Pipitetheropsis and Patis were separated from it. Membership of the group, sometimes considered as a separate subfamily, but more often as a separate clade within the subfamily Pooideae, presently contains 23 genera (GrassWorld 2012): Achnatherum Beauv., Aciachne Benth., Amelichloa Arriaga & Barkworth, Ampelodesmos Link, Anatherostipa (Hack. ex Kuntze) Peñailillo, Anemanthele Veldk. , Austrostipa S.W.L. Jacobs & Everett, Celtica F.M. Vázquez & Barkworth, Hesperostipa (Elias) Barkworth, Jarava Ruiz. & Pav., Macrochloa Kunth, Nassella Desv., Ortachne Nees ex Steud., Oryzopsis Michx, Pappostipa (Speg.) Romaschenko, Peterson & Soreng, Patis O wi, Piptatheropsis Romasch., P.M.Peterson & Soreng, Piptatherum P. Beauv., Piptochaetium Presl, Psammochloa A. Hitchc., Ptilagrostis Griseb., Stipa L., and Trikeria Bor.
Although there has been a concentration of work in specific parts of the world, in particular North America (Barkworth 1990, 1993), Australia (Jacobs & Everett, 1996), south west Asia (Freitag, 1975, 1985) and Asia (Gonzalo et al. 2011) there is no published work that includes a classification of all the genera listed, although Romaschenko et al. (2011) includes all genera except Anemanthele. Keys have been published that discriminate between some of the genera, but the lack of easily discernible morphological characters would explain a trend by some workers not to accept some genera. For example Clayton and Renvoize (1986), who only include 9 genera in the tribe Stipeae, place the genera Jarava, Achnatherum. Macrochloa, Ptilagrostis and Anemanthele in synonymy with Stipa, and present Kew researchers are also reluctant to accept Austrostipa (Cope 2008.). A molecular phylogeny of the tribe Stipeae (Jacobs et al. 2007) shows good support for the monophyly of only three genera - Nassella, Hesperostipa and Piptochaetium. However the recent molecular work of Romaschenko et al. (2008, 2008a) has seven major lineages in the tribe, and that of Barber et al. (2009) finds a problem distinguishing Nassella from Jarava based on molecular data. These papers indicate that the resolution of generic boundaries in the Stipeae is some way from being resolved. Although these authors discuss some morphological characters the latter appear to be of secondary consideration and they do not provide diagnostic morphological keys to the lineages. A new genus Pappostipa with 23 species is elevated from Stipa subg. Pappostipa (Romaschenko et al., 2008) without a clear indication as to how the genus differs morphologically from Stipa.
Simon & Jacobs 1990
Stipoids ({Romaschenko, Monots 2008)
Bra Brachyelytreae Bry Brachypodeae Bro Bromeae Dia Diarrheneae Mel Meliceae Nar Nardeae
Pha Phaeospermateae Po Poeae St Stipeae Tri Triteae
Sti Achnatherum
Sti Aciachne
Tri Aegilops
xAgropogon
Tri Agropyron
Sti Amelichloa
Po Agropyropsis
Po Agrostis
Po Aira
Po Airopsis
Po Alopecurus
Po xAmmocalamagrostis
Po Ammochloa
Po Ammophila
Sti Ampelodesmos
Po Amphibromus
Sti Anatherostipa
Po Ancistragrostis
Sti Anemanthele
Po Aniselytron
Pha Anisopogon
Mel Anthochloa
Tri Anthosachne
Po Anthoxanthum
Po Antinoria
Po Apera
Po Aphanelytrum
Po Arctagrostis
Po Arctophila
Po Arrhenatherum
Tri Australopyrum
Austrofestuca
Sti Austrostipa
Avellinia
Po Avena
Po Avenella
Po Beckmannia
Bro Boissiera
Bra Brachyelytrum
Bry Brachypodium
Po Briza
Po Bromidium
Bro Bromus
Brylkinia
Po Calamagrostis
Po Calotheca
Tri Campelostachys
Po Castellia
Po Catabrosa
Po Catapodium
Sti Celtica
Po Chaetopogon
Po Chascolytrum
Po Cinna
Po Coleanthus
Po Colpodium
Tri Connorochloa
Po Cornucopiae
Po Corynephorus
Tri Crithopsis
Po Cutandia
Po Cyathopus
Po Cynosurus
Po Dactylis
Tri Dasypyrum
Deschampsia
Po Desmazeria
Po Deyeuxia
Di Diarrhena
Po Dichelachne
Po Dielsiochloa
Dissanthelium
Tri Douglasdeweya
Po Dryopoa
Po xDupoa
Po Dupontia
Pha Duthiea
Po Echinaria
Po Echinopogon
Tri xElyhordeum
Tri Elymus
Tri Elytrigia
Po Eremopoa
Tri Eremopyrum
Erianthecium
Euthryptochloa
Po Festuca
Tri Festucopsis
Po Gastridium
Po Gaudinia
Mel Glyceria
Po Graphephorum
Gymnachne
Po Hainardia
Po Helictotrichon
Tri Henrardia
Sti Hesperostipa
Tri Heteranthelium
Po Hierochloë
Po Holcus
Po Hookerochloa
Tri Hordelymus
Tri Hordeum
Po Hypseochloa
Tri Hystrix
Sti Jarava
Tri Kengyilia
Po Koeleria
Po Lachnagrostis
Po Lagurus
Po Lamarckia
Po Leptophyllochloa
Po Leucopoa
Tri Leymus
Po Libyella
Po Limnas
Po Limnodea
Po Lindbergella
Bro Littledalea
Po Loliolum
Po Lolium
Mel Lycochloa
Nar Lygeum
Sti Macrochloa
Po Megalachne
Melica
Pha Metcalfia
Po Mibora
Microbriza
Po Micropyropsis
Po Micropyrum
Po Milium
Po Narduroides
Nar Nardus
Sti Nassella
Po Nephelochloa
Po Nicoraepoa
Po Oreochloa
Sti Ortachne
Sti Oryzopsis
Sti Pappostipa
Po Parafestuca
Po Parapholis
Tri Pascopyrum
Sti Patis
Po Pentapogon
Po Periballia
Tri Peridictyon
Po Peyritschia
Pha Phaenosperma
Po Phalaris
Po Phippsia
Po Phleum
Po Pholiurus
Sti Piptatherum
Sti Piptatheropsis
Sti Piptochaetium
Mel Pleuropogon
Po Poa
Po Podagrostis
Podophorus
Poidium
Po Polypogon
Sti Psammochloa
Tri Psathyrostachys
Pha Pseudodanthonia
Tri Pseudoroegneria
Pseudosclerochloa
Po Psilurus
Sti Ptilagrostis
Po Puccinellia
Po Relchela
Po Rhizocephalus
Rhombolytrum
Po Rostraria
Po Saxipoa
Po Schedonorus
Mel Schizachne
Po Sclerochloa
Po Scolochloa
Po Scribneria
Tri Secale
Po Sesleria
Po Simplicia
Pha Sinochasea
Sitanion
Po Sphenopholis
Po Sphenopus
Tri Stenostachys
Pha Stephanachne
Sti Stipa
Mel Streblochaete
Po Sylvipoa
Sti Taeniatherum
Tri Thinopyrum
Po Torreyochloa
Po Tovarochloa
Sti Trikeraia
Mel Triniochloa
Po Triplachne
Po Trisetaria
Po Trisetum
Tri Triticum
Tzvelevia
Po Vahlodea
Po Ventenata
Po Vulpia
Po Vulpiella
Po Wangenheimia
Po Zingeria
