Fig. 2 from Morrone et al, Phylogeny of the Paniceae (Poaceae: Panicoideae): integrating plastid
DNA sequences and morphology into a new classification. Cladistics 28: 1-24 (2012)
Panicoideae
This subtribe of 203 genera and 3603 species has been traditionally subdivided to the two major tribes Paniceae and Andropogoneae, the former representing about 32% of the subfamily and the latter about 68% (Simon, 2007b). The two tribes are traditionally separated on the basis of a number of diagnostic morphological characters. The Andropogoneae have paired spikelets, with one sessile and usually bisexual and with a geniculate and hairy awn, and the other pedicelled, usually staminate and arranged in modified racemes known as rames. The Paniceae usually have spikelets that are not paired, or if they are, they are generally both pedicelled, sometimes unequally, and similar in sexuality; if awns are present in the Paniceae they are not geniculate and hairy. Although the sets of characters distinguishing Andropogoneae from Paniceae appear characteristic they are not completely unique (synapomorphic), as they are known to occur in at least some Paniceae (Matthews et al. 2002).
Paniceae
Cladistic analyses of the subfamily Panicoideae using the ndhF chloroplast gene (Giussani et al. 2001; Aliscioni et al. 2003; Zuloaga et al. 2007; Morrone at al. 2012) cuts across this traditional classification. In the first and last study the subfamily is shown to consist of two clades, one of which comprises the traditional Andropogoneae and the Paniceae with x = 10 as sister groups and the other clade comprises the Paniceae with x = 9., although in Zuloaga et al. 2007 the strict consensus tree of the panicoid grasses produces a topologyof three lineages – Andropogoneae, Paniceae x=10 and Paniceae x=9. Whatever shape the final topology may have, the composition of the genus Panicum s.l. with about 450 species and once regarded as the world’s largest grass genus, has undergone considerable taxonomic modification in recent times. It appears that this trend will continue, as classification becomes more aligned with phylogenetic clades. Genera that have been removed from Panicum in recent times include Steinchisma Raf. (Zuloaga et al., 1998), Phanopyrum (Raf.) Nash (Aliscioni et al., 2003),Dichanthelium (A. Hitchc. & Chase) Gould (Gould & Clark, 1979), Megathyrsus (Pilg.) B.K. Simon & S.W.L. Jacobs (Simon & Jacobs, 2003),Zuloagaea Bess (Bess et al., 2006) and Hopia Zuloaga & Morrone (Zuloaga et al., 2007). There are at least 16 sections of Panicum of Zuloaga (1987) that lie outside the monophyletic clade of Panicum s.s. and presumably these are all candidates for further taxonomic recognition as separate genera.The most recent study (Morrone et al. 2012) has some of these splits from Panicum as prospective new genera under a new tribe Paspaleae.
If future classifications are to reflect phylogeny, based more on on lineages derived from molecular data, the resulting taxonomic and nomenclatural changes will become a cause for concern. In the panicoid grasses, where generic circumscription based on observable diagnostic differences has been the normal situation until recently, this issue is of particular relevance. For example the consensus tree of the subfamily Panicoideae using the ndhF chloroplast gene (Zuloaga et al., 2007) well illustrates this point. A new monotypic genus Hopia is described from within the Paniceae x = 10 lineage on the basis of its position on a consensus tree, using the ndhF chloroplast gene. Although morphological features of the genus are discussed, these appear to have been of secondary consideration to the selection of this species as a new genus on the basis of its position on a cladogram. For users to be able to recognize this genus surely it would be helpful to show how the new genus differs diagnostically from other genera. Although it is a sister taxon to Paspalum in the cladogram and was previously placed with Panicum, no morphological Latin diagnosis comparing Hopia with these genera is given, but only a Latin description, which in itself, can be read as a generic description of Panicum. Furthermore the table showing a comparison of Hopia with related genera shows no diagnostic characters. In the same paper there are a number of cases of different genera appearing in a single clade. For example there is a clade within the basal lineage of Fig. 2 that has species belonging to five different currently recognised genera – Moorochloa, Melinis, Megathyrsus, Chaetium and Eriochloa. All of these genera have unique morphological attributes that distinguish them from other genera, but if the cladogram were to be considered as a basis upon which to group them under one name, the morphological differences by which these taxa are presently distinguished become of secondary importance to their position on a molecularly based cladogram. Diagnostic characters of use to flora writers would then become extremely difficult to select and for the end user to interpret.
The recently established genus Megathyrsus (Simon & Jacobs, 2003) was established as being separate from Urochloa, with which it had been placed on both morphological (Webster, 1987) and molecular (Aliscioni et al., 2003) grounds. This was done on the basis of it having a very different inflorescence structure than species traditionally placed in Urochloa. The establishment of Megathyrsus has been supported by other morphological and developmental characters – different pattern of initiation of primary inflorescence branches, direction of branch differentiation and phyllotaxis (Reinheimer et al. 2005).
Within the Paniceae genera other than Panicum are also being subdivided based on phylogenetic work, two recent examples being the new genera Oncorachis Morrone & Zuloaga and Keratochlaena Morrone & Zuloaga separated from Streptostachys Desv. (Sede et al. 2009, Morrone & Zuloaga 2009) and Rupichloa Salariato & Morrone separated from Urochloa P. Beauv. (Salariato et al. 2009). In both these papers morphological and molecular characters complement each other in the phylogenetically based classification and in the case of Rupichloa a key to the new genus and its related genera is presented. I feel this should be a manditory step in the process of conveying the generic concept and circumscription of the author to the reader.
Within the x = 9 panicoid clade there have been published in recent times some elegant results on the phylogeny of the “bristle clade” grasses (Bess et al. 2003; Bess et al. 2006; Doust & Kellogg 2002; Donaldio et al. 2009; Doust & Kellogg 2002a; Doust et al. 2007; Kellogg et al. 2004). This group consists of 25 genera of which the three most species rich genera are Setaria P. Beauv., Pennisetum Rich. and Cenchrus L.They form a monophyletic group on the basis of morphological data (Zuloaga et al. 2000) and molecular data from both chloroplast (Bess et al. 2006; Donaldio et al. 2009; Doust & Kellogg 2002a; Doust et al., 2007) and nuclear (Doust et al., 2007) data; the morphological synapomorphy is the presence of setae or bristles in some or all inflorescence branches. Within the topology of this group it transpires that of the above three large genera, Cenchrus is monophyletic (in all papers except Donaldio et al., 2009), Pennisetum paraphyletic and Setariapolyphyletic. On this basis Cenchrus and Pennisetum have been merged under Cenchrus (the older name), although there still needs to be 24 new combinations made for those species still in Pennisetum.
A further puzzle in the “bristle clade” panicoid grasses is reflected in the grass genus Zuloagaea Bess (Bess et al. 2007). On the basis of molecular work (chloroplast ndhF and trnL) this genus is nested within the bristle clade, but at no stage does it show evidence of bristle development. It is sister to the monotypic genus Ixophorus Schlechtd., but it has no morphological synapomorphic character with this genus. In their discussion Bess et al. (2007) even suggest the possibility of expanding the genus Setaria to accommodate Ixophorus and Zuloagaea and furthermore even the genera Spinifex L., Zygochloa S.T.Blake, Pennisetum and Cenchrus, with the comment that “such a radical solution would be cladistically defensible but taxonomically undesirable.” This remark essentially illustrates the dichotomy between evolutionary taxonomy and phylogenetic systematics and the practicality in recognising paraphyletic taxa in certain circumstances (Brummitt 2003, 2006). On a morphological basis the genera Setaria and Paspalidium Stapf appear to form a continuum of variation in different geographic regions, to the extent that species of Paspalidium have been placed in synonymy with Setaria (Jessop et al. 2006 for South Australia; Veldkamp 1994 for Southeast Asia and Webster 1995 for Australia). However the monophyly of the combination of all species of these genera from molecular data was rejected (Kellogg et al. 2009), so they are both retained for the time being.
The morphological characters of the tribe Paniceae have been examined critically within the framework of a DELTA dataset by Webster for different regions of the world (Webster 1987 for Australia; Webster 1988 for North America; Webster & Reyna 1988 for Central America; Webster et al. 1989 for the New World; Webster 1992 for the Old World), with a summary and an analysis of the characters used overall for 102 genera that he recognised (Webster 1992a). Although he had plans to extend his phenetic based studies to a cladistic level (Webster 1992) this did not eventuate; however a lot of this work has now been undertaken, although almost entirely at a molecular level (Giussoni et al. 2000; Aliscioni et al. 2003; Doust et al. 2002; Bess et al. 2005; Bess et al. 2006; Doust et al. 2007; Kellogg et al. 2003; Zuloaga et al. 2006; Zuloaga et al. 2007; Morrone et al. 2012). It remains to be seen how far new circumscriptions of genera based on molecular cladistics will be taken. Although morphological parameters of newly circumscribed genera will able to be produced in DELTA format using the gesummprogram of Dallwitz (2007 onwards) the genera may not have diagnostic morphological characters. Another thorough treatment of the Paniceae at generic level for the Southeastern United States (Crins 1991) presents an excellent review of the 22 genera of Paniceae that occur within the region. Much information is presented on each genus in a worldwide context and all genera have their own list of bibliographic references as well as there being one for the tribe. Suggested relationships to other genera as well as sectional divisions of large genera are presented. At that time not much phylogenetic work had been undertaken on the tribe, with the only cladistic reference being the early morphological work of Kellogg and Campbell (1987). However the essay is an excellent model that could profitably be repeated again, this time for the complete tribe, with the benefit of an extra 20 years of phylogenetic research.
Classification
Incertae sedis Echinochloa Hylebates Sacciolepis Walwhalleya Anthephorinae Anthephora Chaetopoa Chlorocalymma Digitaria Stereochlaena Taeniorhachis Tarigidia Dichantheliinae Dichanthelium Melinidinae Chaetium Eccoptocarpha Eriochloa Louisiella Megathyrsus Melinis Moorochloa Oryzidium Ruphloa Scutachne Thuarea Trholaena Urochloa Yvesia Microcalaminae ( Acroceras Alloteropsis Ampharpum Cyphochlaena Entolasia Lasiacis Microcalamus Oplismenus Ottochloa Parodiophyllochloa Poecilostachys Pseudechinolaena) Neurachninae Ancistrachne Calyptochloa Cleistochloa Neurachne Paraneurachne Thedachloa Thyridolepis Panicinae Arthragrostis Panum Yakirra Setariinae Alexfloydia Camusiella Cenchrus Chamaeraphis Dissochondrus Holcolemma Hygrochloa Ixophorus Paractaenum Paratheria Paspalidium Plagiosetum Pseudochaetochloa Pseudoraphis Setaria Setariopsis Snowdenia Spinifex Stenotaphrum Streptolophus Uranthoecium Whiteochloa Xerochloa Zuloagaea Zygochloa
Paspaleae
This is a new tribe in the subfamily and applies to the mainly American panicoid grasses with the haploid chromosome compliment (x) = 10. When Reynaudia is removed from the group, the remaining genera form a highly supported monophyletic group, and is divided into three major subclades corresponding to the subtribes Arthropogoninae, Otachyrinae and Paspalinae (Morrone et al. 2012). There are also a few genera which, at this stage, do not belong to any of these groups (Incertae Sedis).
Classification
Incertae Sedis Acritochaete Homopholis Hydrothauma Megaloprotachne Thyridachne Trachys, Reynaudia Arthropogonine Achlaena Apochloa Arthropogon Canastra Cyphonanthus Homolepis Keratochlaena Mesosetum Oncorachis Oplismenopsis Phanopyrum Stephostachys Tatianyx Triscenia Otachyriinae Anthaenantia Dallwatsonia Hymenachne Otachyrium Plagiantha Steinchism "Panicum" sect. Laxa Paspalinae Acostia Anthaenantiopsis Axonopus Baptorhachis Centrochloa Echinolaena Gerritea Hopia Ichnanthus Lecomtella Ocellochloa Ophiochloa Paspalum Reimarochloa Renvoizea Spheneria Streptostachys Thrasyopsis
Centothecoid Group
Although this group has been previously recognised and classified as a separate subfamily Centothecoideae, it has only very recently been subsumed within the Panicoideae (Sanchez-Ken & Clark 2010). In GPWG II (2011) all the genera that were previously assigned to the Centothecoideae, together with the tribe Tristachyideae (Chandrasekharania, Danthoniopsis, Gilgiochloa, Jansenella, Loudetia, Loudetiopsis, Trichopteryx and Tristachya - all formerly placed in the tribe Arundinelleae), are grouped together in a lineage named “Outlying Panicoideae”. It was originally described (as Centostecoideae) (Soderstrom 1981) on the basis of discernible characters found in the embryo, lodicules and chromosomes, first noticed earlier by previous workers (Jacques-Felix 1962; Reeder 1957).
In terms of the genera included in the group, opinions have varied but a fairly recent consensus (Davidse 2003; Soriano et al. 2007; Sanchez-Ken & Clark 2007) has 10 pantropical genera (Bromoniola Stapf & C.E. Hubb., Centotheca Desv., Chasmanthium Link, Chevalierella A. Camus, Lophatherum Brongn., Megastachya P. Beauv., Orthoclada P. Beauv., Pohlidium Davidse, Soderstr. & R.P. Ellis, Thysanolaena Nees, Zeugites P. Browne. Five are monotypic (Bromoniola, Chevalierella, Megastachya, Pohlidium, Thysanolaena), two are ditypic (Lophatherum, Orthoclada); of the remaining three, Centotheca has 4 species, Chasmanthium has 6 species and Zeugites has 11 species. Sanchez-Ken & Clark (2007) have two other monotypic genera Gouldochloa Valdés-Reyna, Morden & S.L. Hatch and Calderonella Soderstr. & H.F. Decker but they have been placed in synonymy with Chasmanthium and Zeugites respectively (Davidse 2003; Soriano et al. 2007).
The most recent work on this group (Sanchez-Ken and Clark 2010) restricts the tribe Centotheceae to Bromoniola, Centotheca, Chevalierella, Megastachya and Pohlidium. Other small genera are assigned to separate tribes on the basis of phylogenetic analysis - Chasmanthieae (Chasmanthium), Cyperochloeae (Cyperochloa and Spartochloa), Gynerieae (Gynerium), Hubbardieae (Hubbardia), Steyermarkochloeae (Arundoclaytonia and Steyermarkochloa) and Thysanolaeneae (Thysanolaena).
Classification
Centotheceae Bromoniola, Centotheca, Chevalierella, Megastachya Pohlidium Chasmantheae Chasmanthium Thysanolaeneae ThysanolaenaZeugiteae Lophatherum, Orthoclada, Zeugites
Distribution of Centhecoid group (Sanchez-Ken pers. com.)
Three tribes that have been excluded from the Centhecoideae group (Sanchez-Ken & Clark 2007), but are obviously are closely related (Morrone et al 2012) are the three small tribes Cyperochloeae (Cyperochloa and Spartochloa), Gynerieae (Gynerium) and Steyermarkochloeae(Arundoclaytonia and Steyermarkochloa. Also allied is Tristachyideae (Chandrasekharania, Danthoniopsis, Gilgiochloa, Jansenella, Loudetia ,Loudetiopsis, Trichopteryx, Tristachy), a new tribe of panicoid grasses that included genera that used to belong to the Arundinelleae
Andropogoneae
The pantropic mainly Old World savanna tribe Andropogoneae has been shown to be monophyletic (Clayton 1987; Kellogg & Watson 1993; Kellogg 2000), with distinguishing characters of unevenly paired spikelets arranged in rames, the sessile spikelets bisexual and the pedicelled spikelets usually staminate, the spikelet pair usually disarticulating with a segment of the rachis and the pedicel and having a C4 NADP-ME photosynthetic pathway. However the phylogeny and classification of the tribe, consisting of 85 to 100 genera (Clayton & Renvoize, 1986; Watson & Dallwitz, 1992) continues to present problems. The original classification based on morphology (Hackel, 1889) was largely unchanged in later classifications (Keng, 1939, Clayton & Renvoize, 1986), although many subgenera of Andropogon were elevated to generic rank. It was divided to 11 subtribes by Clayton (1972a and 1973), with the first five consisting of awned genera (Clayton, 1972a) and the second three of awnless genera (Clayton 1973). Watson & Dallwitz (1992) had two subtribes, the Andropogoninae (the awned genera) and Rottboelliinae (the awnless genera equivalent to Clayton’s first awnless tribe); the genera of Clayton’s other two awnless genera were placed in the tribe Maydeae, following earlier classifications. In a phylogenetic study based on morphology and that included the Arundinelleae (Kellogg & Watson, 1993) it was demonstrated that none of Clayton’s subtribes is monophyletic.
Molecular cladistic work to date in the tribe Andropogoneae (Kellogg 2000; Spangler 2000; Matthews 2002; Skendzic et al. 2007; Teerawatananon & Hodkinson 2008) shows some results that conflict with the traditional classifications based on morphology, although sample size is still rather meagre. In three molecular studies cited in Kellogg (2000), Andropogoneae is shown to be monophyletic only if Arundinella is included, differing from the result obtained from morphological cladistics (Kellogg & Watson, 1993), where Arundinella is placed within a monophyletic Arundinelleae. On the basis of the molecular results it has been proposed this genus be included in the tribe Andropogoneae(Kellogg, 2000), although it’s paired spikelets are not of the sessile - pedicelled type as met with in true andropogonoids. Furthermore on molecular evidence it is recommended that Tristachya be placed with the Andropogoneae and Danthoniopsis with the Paniceae (Kellogg, 2000).
There have recently been two molecular studies on the Sorghum group in Australia that have produced slightly different results. One study (Spangler et al. 1999; Spangler 2000; Spangler 2003), using ndhF sequences, produced a phylogeny of three clades for the ‘Sorghum’ taxa, for which the names Sorghum Moench, Sarga Ewart & White and Vacoparis Spangler were assigned. A subsequent study (Dillon et al. 2001; Dillon et al. 2004; Price et al. 2005), using combined ITS1 ndhF sequences produced a phylogeny of two clades, with S. bicolor appearing in the same clade as the species assigned to the genus Vacoparis by Spangler. While the genus Sarga is corroborated by the second study, it appears that the genus Vacoparis may have been established rather prematurely and is perhaps an example where more caution should have exercised before establishing a new classification.
Within the Andropogoneae the subtribe Rottboellinae is recognised phenetically in traditional classifications (Hackel 1889; Stapf, 1917; Clayton 1973; Clayton & Renvoize 1986) on the basis of the rachis internodes and pedicels being stout and thickened upwards and more or less fused, the spikelet pairs being heterogamous, with the pedicelled either reduced or absent and the upper lemma being awnless. It is composed of a number of small genera, the circumscription of which has been the subject of some controversy (Clayton 1973; de Koning et al. 1983; Veldkamp et al. 1986); when all genera that have been placed in the subtribe are listed they include Chasmopodium Stapf, Coelorachis Brongn., Elionurus Humb. & Bonpl., Eremochloa Buese, Glyphochloa W.D.Clayton, Hackelochloa Kuntze, Hemarthria R.Br., Heteropholis C.E.Hubb., JardineaSteud., Lasiurus Boiss., Loxodera Launert, Manisuris L., Mnesithea Kunth, Ophiuros Gaertn.f., Oxrrhachis Pilger, Phacelurus Griseb., PseudovossiaA.Camus, Ratzburgia Kunth, Rhytachne Desv., Robynsiochloa Jacques-Félix, Rottboellia L.f., Thaumastochloa C.E.Hubb., Thyrsia Stapf, UrelytrumHackel and Vossia Wall. & Griff.
In the phenetic analysis of Clayton (1973) 43 characters are used to produce graphic representation of the degree of similarity of 98 species belonging to all of the above genera excluding Robynsiochloa and including Tripsacum laxum Nash of the Tripsacinae. The minimum spanning tree best shows the relationships of the genera and species, arranging them into 5 groups. No attempt has been made to undertake a morphological cladistic study of the group using these 43 characters, although a subset was used in an early cladistic study of species ofHeteropholis and Thaumastochloa (de Koning et al. 1983). A further study on the classification of some of the Rottboellinae (excluding theElionurastrae, Lasiurastrae and Vossiastrae of Clayton 1973) (Veldkamp et al. 1986) resulted in a much expanded circumscription of the genusMnesithea that included Heteropholis, Rottboellia formosa, Coelorachis and Hackelochloa. The rationale for this treatment was posed by the dilemma of the authors as to what exactly defines a ‘good’ genus, whatever that means. They believe that “the generic concept must be a compromise between biological reality and practical convenience.” The basis for this merging of genera appears to be based on the subsuming of paraphyletic taxa by means of a discussion of morphological characters of some species, without the presentation of a cladogram. They talk about a “botanical black hole” where “genera around the complex spiral down into it” much in the same way that later became the centre point for the discussion on the need to accept paraphyletic taxa (Brummitt 2002, 2003, 2006; Nordal & Stedje, 2005).
Other cladistic work on the Rottboellinae - morphological (Kellogg & Watson 1993) and molecular (Skendzic et al., 2007) – indicate the subtribe is not monophyletic. However the morphological results shows that only 3 genera, Elionurus, Oxyrachis and Sehima, fall outside a clade that includes all the other genera. Molecular cladistic work so far has been restricted to two species of Elionurus, and one each of Hemarthria andHackelochloa, within a study that sampled fairly thinly in the Andropogoneae; a clade of these four species and Coix lacrma-jobi of the related subtribe Coicinae was produced. Much wider sampling is needed to obtain more meaningful results and publication of more recent work (Teerawatananon & Hodkinson, 2008) is awaited for possible clarification.
Classification
Incertae Sedis Chasmopodium Chionachne Chrysopogon Eremochloa Garnotia Polytoca Trilobachne Andropogoninae Agenium Anadelphia Andropogon Arthraxon Bhidea Bothriochloa Capillipedium Clausospicula Cymbopogon Dichanthium Diheteropogon Euclasta Eulaliopsis Exotheca Heteropogon Homozeugos Hyparrhenia Hyperthelia Elymandra Iseilema Microstegium Monocymbium Parahyparrhenia Pseudanthistiria Pseudodichanthium Schizachyrium Spodiopogon Themeda Trachypogon Urelytrum Arundinellinae Arundinella Germainiinae Apocops Germainia Lophopogon Polytrias Ischaeminae Andropterum Apluda Dimeria Ischaemum Kerriochloa Loxodera Pogonachne Sehima Thelepogon Triplopogon Ophiurus + Oxyrachis + Thaumastochloa Group Ophiuros Oxyrhachis Thaumastochloa Phacelurus Group Elionurus Lasiurus Phacelurus Rhytachne Vossia Rottboelliinae Coix Glyphochloa Hemarthria Manisuris Mnesithea Ratzeburgia Rottboellia Saccharinae Asthenochloa Cleistachne Erianthus Eriochrysis Eulalia Hemisorghum Imperata Miscanthus Pogonatherum Pseudopogonatherum Pseudosorghum Saccharum Sarga Sorghastrum Sorghum Spathia Vacoparis Veldkampia Zea + Tripcacum Clade Tripsacum Zea
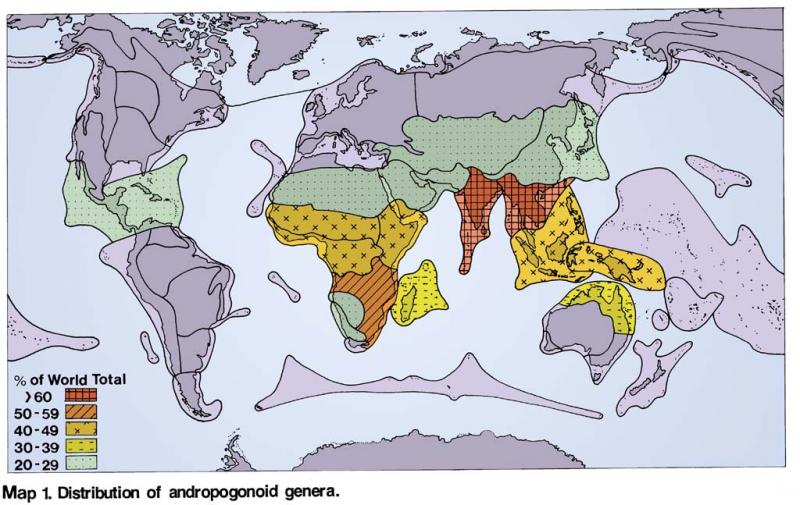
Distribution of Andropogoneae (Hartley 1958) Distribution of Paniceae (Hartley 1958)

Simon & Jacobs 1990
